Applied Sciences Free FullText Barriers and Chemistry in a Bottle
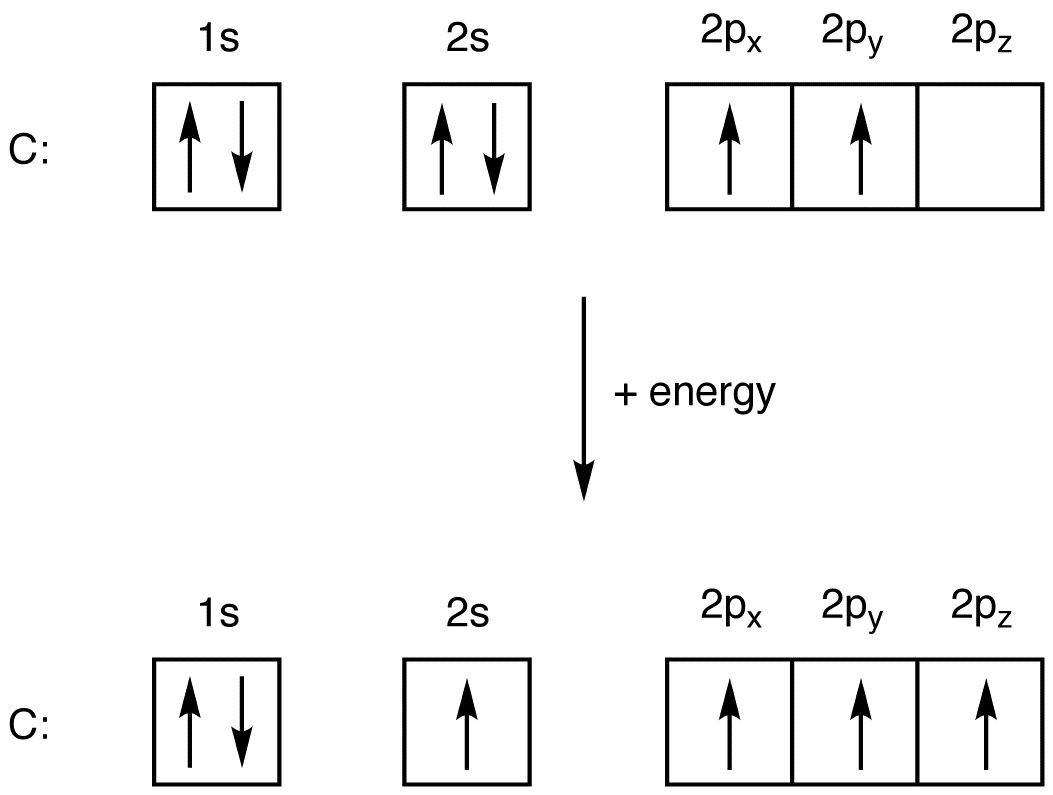
Oxygen is the 8th element in the periodic table and its symbol is 'O'. In this article, I have discussed in detail how to easily write the complete electron configuration of oxygen. What is the electron configuration for oxygen? The total number of electrons in oxygen is eight.
Oxygen Electron Configuration (O) with Orbital Diagram
The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram. The video uses Kr as an example, but the process is exactly as the same as what you need to do for oxygen. Hope this helps!

Oxygen Valence Electrons (O) Oxygen Valency & Electron Configuration
Oxygen is the eighth element with a total of 8 electrons. In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be.

29 Diagram Of Oxygen Atom Wiring Diagram Info
To write the orbital diagram for the Oxygen atom (O) first we need to write the electron configuration for just O. To do that we need to find the number of electrons for the Oxygen atom.

Molecular Orbital Diagrams for O2 101 Diagrams
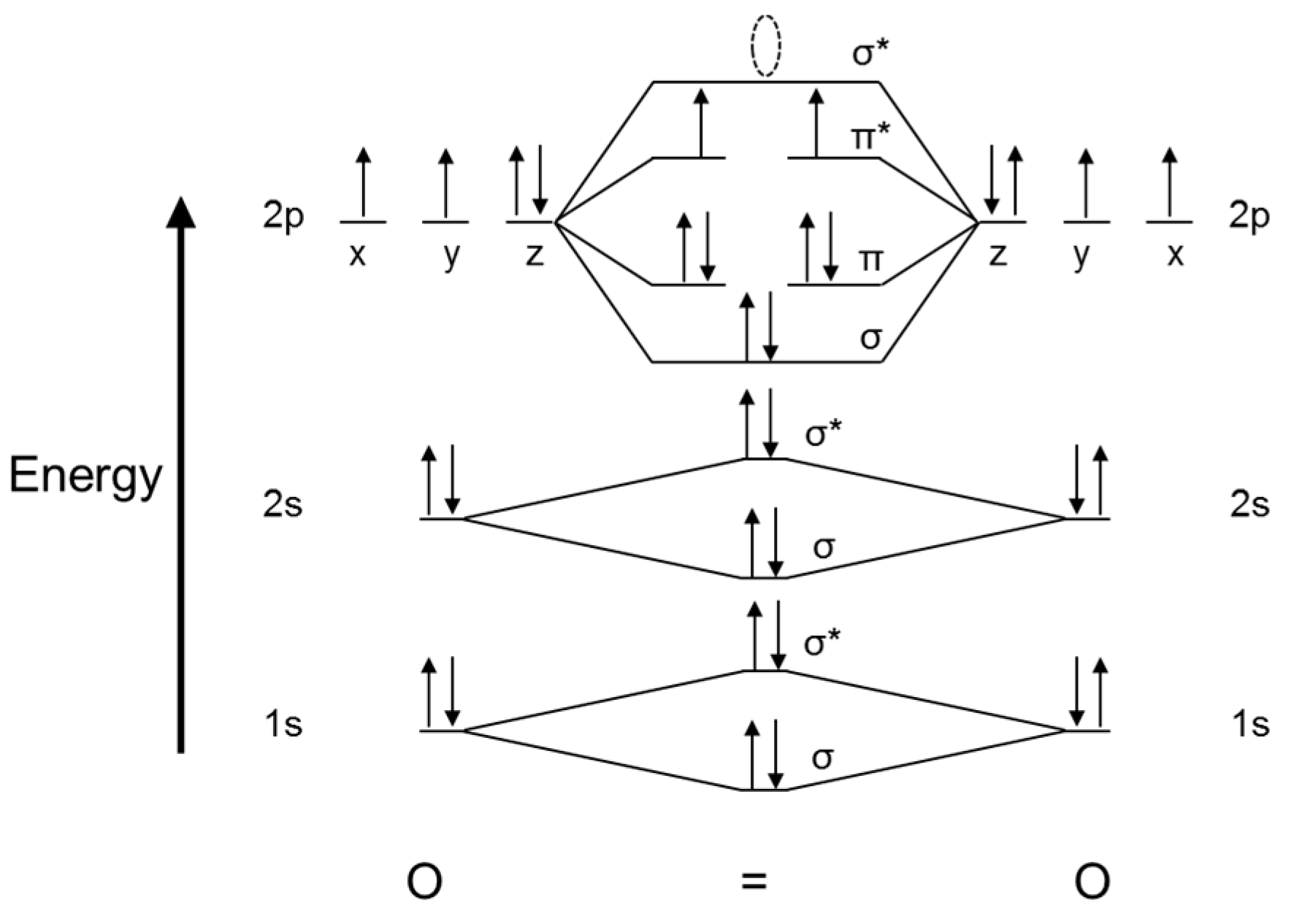
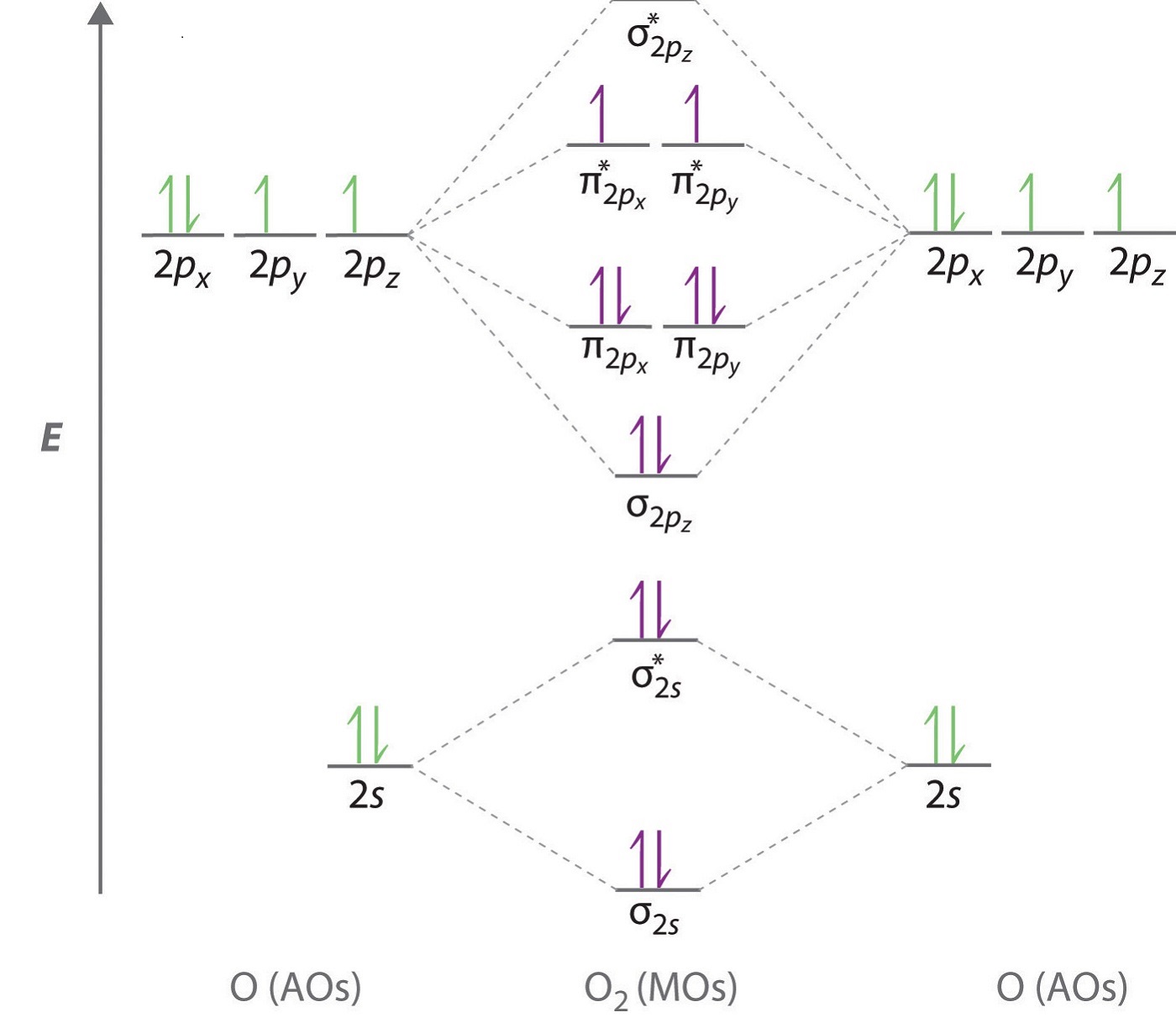
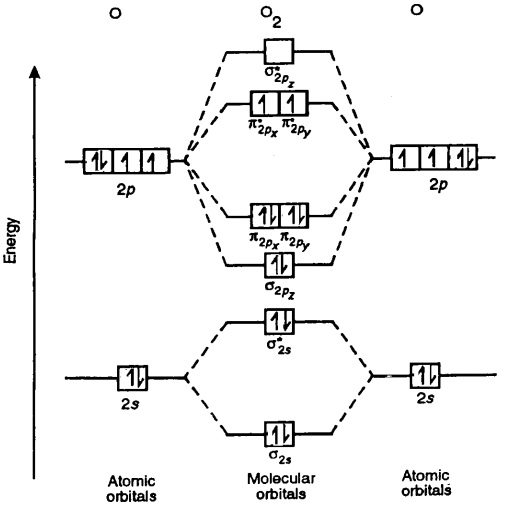
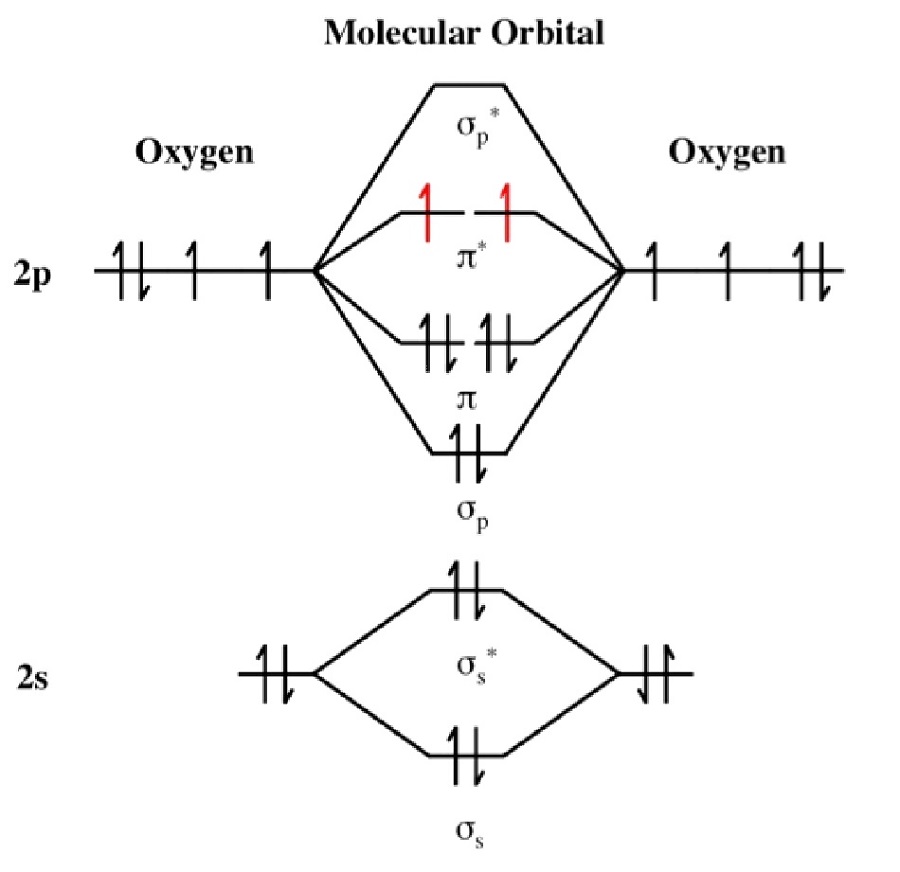
As of December 2014, up to 46% of the energy in sunlight could be converted into electricity using solar cells. Example 6.9.2: M olecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons. Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2.
O2 Molecular Orbital Diagrams 101 Diagrams
In O 2, therefore, we need to accommodate twelve valence electrons (six from each oxygen atom) in molecular orbitals. As you can see from the diagram, this places two electrons in antibonding orbitals. Each of these electrons occupies a separate π* orbital because this leads to less electron-electron repulsion (Hund's Rule).

O2 Molecular Orbital Diagrams 101 Diagrams
From this diagram, calculate the bond order for [latex]\ce{O2}[/latex]. How does this diagram account for the paramagnetism of [latex]\ce{O2}[/latex]? Show Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 7.7.12. Each oxygen atom contributes six electrons, so the diagram appears as shown in Figure 7.7.15.

Schematic of the ‘O2’ molecular orbital diagram. The figure explains
Solution. The bond length in the oxygen species can be explained by the positions of the electrons in molecular orbital theory. To obtain the molecular orbital energy-level diagram for \(\ce{O2}\), we need to place 12 valence electrons (6 from each O atom) in the energy-level diagram shown in Figure 9.10.1 .
Molecular Orbital (MO) Diagram for O2() YouTube
When two oxygen atoms overlap, the sigma (2p) molecular orbital is LOWER in energy than the pi (2p) orbitals. This different from Nitrogen, where it's the othe.

The Orbital Diagram For A Ground State Oxygen Atom Is Hanenhuusholli
Electronic structure Singlet oxygen refers to one of two singlet electronic excited states. The two singlet states are denoted 1 Σ + g and 1 Δ g (the preceding superscript "1" indicates a singlet state). The singlet states of oxygen are 158 and 95 kilojoules per mole higher in energy than the triplet ground state of oxygen.
Oxygen molecule Homework Help Assignment Help Molecular orbital
Oxygen orbital diagram October 20, 2023 by Deep The information on this page is fact-checked. Oxygen orbital diagram The orbital diagram of oxygen shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, and the 2p subshell has 4 electrons. Contents Steps Find electrons Write electron configuration Draw orbital diagram Related
O2 Molecular Orbital Diagrams 101 Diagrams
The left-hand side diagram is of O2 at ground level whereas the right-hand side diagram is of rearranged electrons as per the Lewis structure within the O2 molecule. It takes a lot of energy to pair up the electrons within the same orbital. So, the diagram having no unpaired electrons is at higher energy. It means it is at a much higher excited.

Solved Complete the valence molecularorbital diagram for
With knowledge of both orbital symmetries and energies, we can construct the molecular orbital diagram. The valence orbitals of oxygen go on one side of the diagram while the hydrogen group orbitals are drawn on the opposite side. Molecular orbitals are drawn in the center column of the diagram, as shown in Figure \(\PageIndex{3}\): Figure.
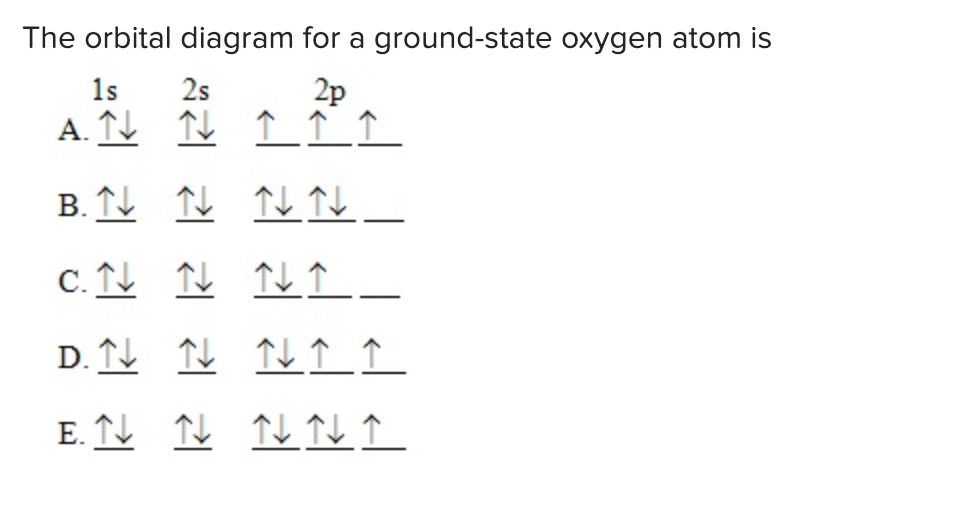
Solved The orbital diagram for a groundstate oxygen atom is
The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 8.3. 3 or 8.3. 4 ). Thus, the electron configuration and orbital diagram of lithium are:

How do you write the orbital diagram for phosphate? Socratic
An oxygen atom is a neutral atom that has 8 atomic numbers which imply it has a total of 8 electrons. As per the Aufbau rule, the electrons will be filled into 1s orbital first then 2s, then 2p…so on. Now, for the electron configuration of Oxygen, the first 2 electrons will go in 1s orbital since s subshell can hold a maximum of 2 electrons.

What is the Electron Configuration of Oxygen Archives Dynamic
In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of l differ so that the energy of the orbitals increases within a shell in the order s < p < d < f. Figure 6.24 depicts how these two trends in increasing energy relate.